Radiotherapy
Learning Objectives
- Cancer Basics
- Fractionation
- The use of photons in radiotherapy
- Special techniques in radiotherapy
- Role of physicists in radiotherapy
Radiotherapy is the treatment of cancer with ionising radiation.
What is cancer?
The abnormal, uncontrolled growth of cells that ultimately forms a tumour. As the tumour grows, some abnormal cells can break off and spread via the blood and lymph system to other parts of the body. This is known as a metastatic spread.
Stages of Cancer
Cancer is initiated by a genetic mutation that affects the cell growth mechanism. Internal or external agents can promote the growth of that particular cancer. Cancer then progresses and becomes more aggresive
Cancer Treatments
Treatments for cancer can include surgery, chemotherapy, hormone therapy, immunotherapy and radiotherapy.
Chemotherapy
Chemotherapy is where drugs are used to kill rapidly dividing cells. However cancer cells are not the only cells which are quickly dividing, other cells such as Red and White Blood Cells are also killed during treatment. As a result this lowers the immune system of patients, making them prone to infections. Another side effect is hair loss.
Hormone Therapy
Some cancer cells are hormone receptive (e.g. breast cancer). Drugs such as Tamoxifen can bind to these receptor sites on the cancer cells, preventing their further division.
Immunotherapy
Tumour cells bind to T-cells to deactivate them. Immunotherapy drugs specifically block tumour cells from deactivating the T-cells, so the T-cells will kill the cancer cell.
Ionising Radiation Treatment
If an ionisation treatment is applied during mitosis (as the cell is dividing), this is the most effective phase in the cell cycle to damage the cell.
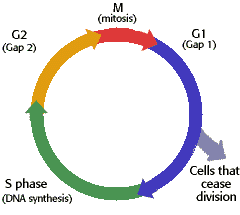
Cancer cells cope less with an increase in radiation dose than normal cells, as cancer cells are more often in metaphase.
Fractionation
This is a form of radiotherapy where multiple small doses of radiation are used at intervals to take advantage of the fact that normal cells recover faster (and are less damaged by radiation) than cancer cells.
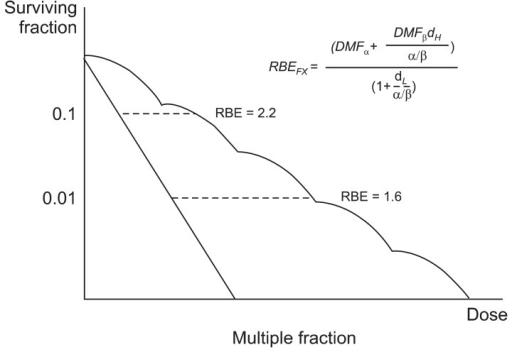
Fractionation has an enhanced effect on cancer cells. The common mode of delivery is around 30 fractions over 6 weeks. Hyperfractionation is an experimental technique using even more fractions.
Radiation Dose Gray
A prescription is given in cGy (centiGray) which is related to former unit, rad (where 100 rad = 1 Gy). Usual doses are given in 10’s Gy in fractions of 1-2 Gy. Accuracy is critical (needs to be above 3%). A typical radiotherapy treatment could be 50 Gy.
Photons
X or gamma rays are predominantly used in radiotherapy. Their penetration increases with energy, and have interactions that include photoelectric absorption, compton scatter and pair production.
Depth Dose Characteristics
The ionisation is greatest not at the surface of the skin but at a slight depth. This slight depth is known as the build up. The higher the energy the longer it takes to ‘build up’ the number of electrons so the depth is deeper.
Plotting tank
Is a large size motorised 3D water phantom for automatic dose distribution measurement of radiation therapy beams. Inside a plotting tank, there is an ionisation chamber to accurately measure the detection and measurement of certain types of ionising radiation; X-rays, gamma rays and beta particles.

Treatment Planning
A treatment is planned to maximise the dose to the tumour and minimise the dose to the surrounding tissue. The parameters than a radiotherapist can change is the penetration, the number of fields, the field size and the field shape. It is usually computerised.
In order to plan a treatment, imaging is often used to locate the tumour. Tumour localisation and beam positioning can be found using a CT simulator. Fluoroscopy and beam positioning can be used to stimulate a treatment.
Single Field Treatment Plan
This is an unsatisfactory treatment plan, as there is a dose gradient across a tumour, and a higher dose is given to the normal tissue above it.
Parallel Opposed Treatment Plan
This is a more accurate, where the same field is given from both sides. However again it is not sufficient to provide a cure because it causes too much damage to normal tissue. It is used almost always in palliative treatment.
External Beam Radiation Therapy
With more beams, the treatment begins to become more accurate and effective than the single/parallel field treatments. However there are still come complexities with treatment. For example, the rectum is next to the prostate, but it is extremely sensitive to radiation. So to avoid any unwanted effects the beam is modified using:
- Collimators
- Filters (beam flattening)
- Wedges
- Compensators
Treatment Delivery
Treatment can be delivered using a variety of techniques:
- Gamma therapy: originally used Radium 226, more recently caesium 137. The problem with gamma is that it has to be changed every few years and so these machines are no longer used.
- Superficial x-ray: (approx 300 keV) is used to treat cancers on or near the skin surface.
- Linear Accelerators: (Linac) these are x-ray devices, using 5 - 25 MeV. It has almost replaced cobalt external beam machines. See below for a cross-section of a linear accelerator.

Alignment
Patient set-up is crucial, with alignment as simulated. Patient’s are often given skin tattoo’s to align them with a laser as accurately as on the simulator. A cast is used for head and neck to prevent the patient from moving. The latest systems are even synchronised to breathing.
Special Techniques
Portal Imaging
This is imaging during therapy. The alignment of the beam is confirmed with the tumour site. This allows modifications to the position etc. on the fly if the tumour volume changes during treatment.
Intensity Modulated Radiation Therapy IMRT
This involves treating irregular shaped tumours. Detailed planning is required. It uses a multileaf and dynamic collimator, which aids in beam shaping/accuracy. Tomotherapy scans a tumour while it is being treated by radiotherapy.
Gamma Knife
This is used to combat brain tumour. It consists of multiple small beams (60+). Complex planning is involved.
Total Body Irradiation TBI
TBI is used in leukaemia treatment. It destroys malignant bone arrow, which is replaced by a transplant of matched donor marrow. The set up is designed for uniform irradiation, lung dose is critical (10 Gy dose).
External Beam Radiotherapy EBRT
External beam radiotherapy (EBRT) or teletherapy is the most common form of radiotherapy (radiation therapy). The patient sits or lies on a couch and an external source of ionizing radiation is pointed at a particular part of the body.
Brachytherapy
Brachytherapy is a form of radiotherapy where a sealed radiation source is placed inside or next to the area requiring treatment. Brachytherapy is commonly used as an effective treatment for cervical, prostate, breast, and skin cancer and can also be used to treat tumours in many other body sites.
Role of Physicists
Physicists are heavily involved in treatment planning, identify problems and providing solutions. During treatment planning they lease with the oncologist and determine the best ‘set-up’. Physicists calculate the point dose and discuss this with the oncologist.
They look at the beam output, field uniformity, beam alignment (radiation/light), interlocks (doors, wedges etc). They measure all field sizes to ensure beam data is correct, beam outputs (all energies, photons and electrons) and other safety features. This process can take months.
Physicists are also commissioned for room design and protection. For example in a radiotherapy bunker, shielding, scattering and safety features all have to be calculated.
Written by Tobias Whetton